"The government will only provide vaccines that are proven safe and have passed clinical trials in accordance with the WHO recommendation," Putranto stated during an online press conference on the arrival of the COVID-19 vaccine from China here on Monday.
COVID-19 handling in Indonesia is not just restricted to applying the 3M protocols of wearing a mask, physical distancing, and washing hands, the minister noted.
The government also deemed it necessary to intervene by breaking the chain of transmission of COVID-19 by providing immunity to the community through vaccination, he expounded.
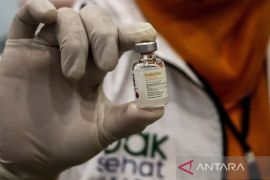
Nearly 1.2 million doses of readied COVID-19 vaccine arrived at the Soekarno-Hatta Cengkareng Airport on Sunday night. Related news: COVID-19 vaccine defect-free and in good condition: Health Minister
Related news: Govt should increase public cognizance of COVID-19 vaccines: House
The health minister noted that delivery of the vaccine was the first phase of procurement of three million doses of COVID-19 vaccines in the form of the inactivated SARS CoV-2 virus.
The Food and Drug Administration (BPOM) is expected to soon issue an Emergency Use Authorization (EUA) concerning the COVID-19 vaccine.
"The vaccine will have to go through the Emergency Use of Authorization (EUA) by the BPOM in accordance with scientific and statutory provisions," he emphasized.
Moreover, Putranto made assurance of the COVID-19 vaccine having arrived in Indonesia being defect-free and ready to be delivered to storage warehouses aboard a refrigerated transport vehicle.
The ministry ensured good condition of the refrigerated transport vehicle, provided by the state-run vaccine manufacturer PT Bio Farma, for monitoring and maintaining the vaccine temperature in accordance with the procedure.
The authority has prepared a storage warehouse, with cold chain management, for 1.2 million doses of the COVID-19 vaccine received from China's Sinovac Biotech Ltd before being distributed to health offices nationwide.
Medical workers and their assistants as well as supporting workers at health facilities will be prioritized in the first batch of COVID-19 vaccination, he revealed.(INE)
Related news: BPOM evaluation is prerequisite for conducting COVID-19 vaccination
Related news: After receiving Sinovac, Indonesia eyes COVAX's vaccines in 2021
Translator: Aditya R, Fardah
Editor: Yuni Arisandy Sinaga
Copyright © ANTARA 2020